 The question regarding benefit of drug eluting stents for high-grade intracranial atherosclerosis has been the topic of several recent investigations. The need is high with upwards of 32% of bare metal stents developing restenosis of >50% and high risk of stroke as reported in Stenting and Aggressive Medical Management for Preventing Recurrent Stroke in Intracranial Stenosis (SAMMPRIS) trial 1. The Vitesse Intracranial Stent Study for Ischemic Stroke Therapy (VISSIT) was also plagued by a high complication rate 2. Drug eluting stents offered a novel approach to addressing some of the initial concerns seen with bare-metal stents. Park and colleagues found in their initial 11 patient cohort that significant restenosis was greatly reduced with drug-eluting stents 3. Qureshi and colleagues further verified that drug-eluting stents were safe and had low rates of major stroke or death 4. Ding and Liu have even proposed indications for use given the various drug eluting stents now available on market 5. Most of the evidence to date has been based on small cohort studies or case series. The indication for a randomized clinical trial was apparent.
The question regarding benefit of drug eluting stents for high-grade intracranial atherosclerosis has been the topic of several recent investigations. The need is high with upwards of 32% of bare metal stents developing restenosis of >50% and high risk of stroke as reported in Stenting and Aggressive Medical Management for Preventing Recurrent Stroke in Intracranial Stenosis (SAMMPRIS) trial 1. The Vitesse Intracranial Stent Study for Ischemic Stroke Therapy (VISSIT) was also plagued by a high complication rate 2. Drug eluting stents offered a novel approach to addressing some of the initial concerns seen with bare-metal stents. Park and colleagues found in their initial 11 patient cohort that significant restenosis was greatly reduced with drug-eluting stents 3. Qureshi and colleagues further verified that drug-eluting stents were safe and had low rates of major stroke or death 4. Ding and Liu have even proposed indications for use given the various drug eluting stents now available on market 5. Most of the evidence to date has been based on small cohort studies or case series. The indication for a randomized clinical trial was apparent.
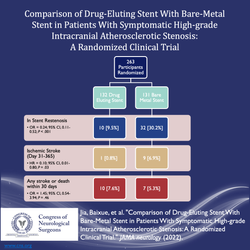
Jia and colleagues evaluated 263 patients who were randomly assigned to receive a drug eluting stent or bare-metal stent. The designed endpoint was in-stent stenosis >50%, which was seen in 9.5% of drug eluting stents verse 30.2% of bare-metal stents. No significant difference in stroke or death was seen within 30 days 6. The number needed to treat for benefit with drug eluting stent was 4.8. Follow up angiography was done at 1 year confirming low in-stent stenosis. Limitations of the study include the short follow up period and single institution design. Given the proposed mechanism of inhibition of endothelial cell proliferation and migration, it would be necessary to evaluate time frames over the course of years 7. Further multi-institutional validation studies are warranted to ensure generalizability.
References
- Abou-Chebl A, Bashir Q, Yadav JS. Drug-eluting stents for the treatment of intracranial atherosclerosis: Initial experience and midterm angiographic follow-up. Stroke. 2005;36:e165-168
- Zaidat OO, Fitzsimmons BF, Woodward BK, Wang Z, Killer-Oberpfalzer M, Wakhloo A, et al. Effect of a balloon-expandable intracranial stent vs medical therapy on risk of stroke in patients with symptomatic intracranial stenosis: The vissit randomized clinical trial. JAMA. 2015;313:1240-1248
- Park S, Lee DG, Chung WJ, Lee DH, Suh DC. Long-term outcomes of drug-eluting stents in symptomatic intracranial stenosis. Neurointervention. 2013;8:9-14
- Qureshi AI, Kirmani JF, Hussein HM, Harris-Lane P, Divani AA, Suri MF, et al. Early and intermediate-term outcomes with drug-eluting stents in high-risk patients with symptomatic intracranial stenosis. Neurosurgery. 2006;59:1044-1051; discussion 1051
- Ding D, Liu KC. Applications of stenting for intracranial atherosclerosis. Neurosurg Focus. 2011;30:E15
- Jia B, Zhang X, Ma N, Mo D, Gao F, Sun X, et al. Comparison of drug-eluting stent with bare-metal stent in patients with symptomatic high-grade intracranial atherosclerotic stenosis: A randomized clinical trial. JAMA Neurol. 2022
- Inoue T, Node K. Molecular basis of restenosis and novel issues of drug-eluting stents. Circ J. 2009;73:615-621